Industrialisation of a breath-actuated inhaler
An asthma treatment for adults and adolescents
Inhaler Industrialisation engineering
Challenge
Working on behalf of Team Consulting to support MundiPharma with the industrialisation and scale-up of a breath-actuated inhaler for the treatment of asthma.
Approach
We worked with Team (device design partners) to provide inhaler industrialisation engineering support to MundiPharma, in order to bring the device to market.
Outcome
The breath-actuated inhaler was launched in Europe in 2018 and is available as an asthma treatment for adults and adolescents.
When Mundipharma went to Team Consulting they already had a developed product, manufacturable from low volume production systems. They required a new device design partner, to be responsible for the device constituent part of their product during the process of industrialisation, scale-up and launch.
For this project Team provided an experienced team of mechanical and production engineers, as well as experts in device testing and verification, and Cambridge Medtech Solutions were asked to join the project team.
Across the whole project, Cambridge Medtech Solutions, with Team supported MundiPharma by:
- Preparation of the Device Functionality Profile in accordance with ISO 20072 and other applicable standards, based on the MundiPharma requirements specification
- Supported the procurement and qualification of multi-cavity production tooling
- Preparation and review of tolerance analyses, mathematical models and engineering analyses.
- Contributions to risk management activities.
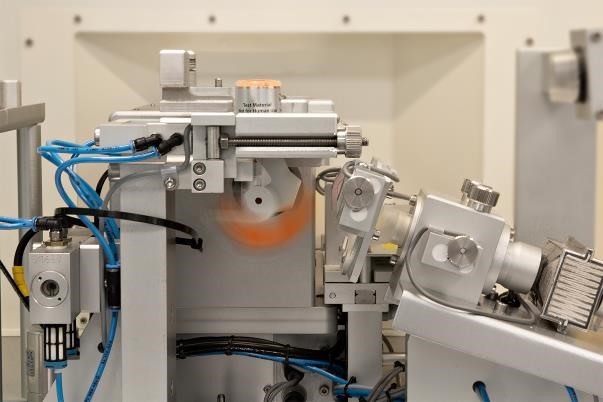
- Management, specification, review and commissioning of bespoke automated test equipment to enable high-throughput functionality testing of the inhalers.
- Management, specification, review and commissioning of bespoke manual pharmaceutical test equipment.
The breath-actuated inhaler was launched in Europe in 2018 and is now commercially available.