Acrylamide Reduction
Reducing Carcinogenic and Neurotoxic Risks
Market Research, Go-To-Market Strategy, Pilot Trials
Challenge
Acrylamide is a chemical that can form in certain foods during high-temperature processing. Scientific evidence has linked acrylamide exposure to increased long-term health risks, including a higher likelihood of cancer and potential effects on the nervous system. As awareness and regulatory scrutiny grow, the client needed a practical, evidence-based way to reduce acrylamide formation without compromising product quality, safety, or commercial viability.
The challenge was threefold:
- Understand market expectations and regulatory pressures around acrylamide reduction
- Define a clear path to commercialisation for an acrylamide-reduction solution
- Validate the technical effectiveness of the approach under real-world conditions
Approach
Cambridge Medtech Solutions delivered the project in three integrated phases:
1. Market Research
- Assessed global regulatory guidelines, industry benchmarks, and emerging best practices related to acrylamide reduction
- Interviewed key stakeholders across manufacturing, quality, and regulatory functions
- Evaluated customer willingness to adopt new technologies aimed at improving food safety and public health outcomes
2. Go-to-Market Strategy Development
- Defined the value proposition around health protection, regulatory compliance, and brand trust
- Identified priority market segments and early adopters
- Developed a phased commercialisation roadmap aligned with manufacturing and regulatory timelines
3. Pilot Trials
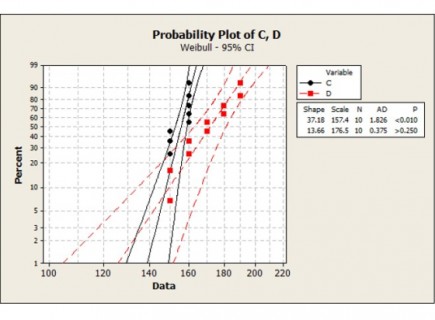
- Designed and executed pilot trials to test acrylamide-reduction performance in representative production conditions
- Collected and analysed data to quantify reductions while monitoring taste, texture, and process efficiency
- Refined technical and operational requirements to support scale-up
Outcome
- Demonstrated measurable acrylamide reduction in pilot trials, supporting the solution’s role in improving consumer health outcomes
- Delivered a robust, data-driven go-to-market strategy that aligned health benefits with commercial and regulatory needs
- Enabled the client to move forward confidently toward scale-up and market launch, positioning the solution as a proactive response to public health concerns and evolving regulations
By addressing acrylamide reduction through both technical validation and strategic planning, the project helped bridge the gap between health science, technology development, and real-world adoption.