Lyophilised Product and Packaging
Robustness and Usability Comparability Study
Reliability Engineering, MEOST, Weibull Analysis, User Interaction
Lyophilisation or Freeze-Drying is a dehydration process typically used to preserve a perishable material, or make the material more convenient for transport or use. This technique is used on a range of healthcare products; both prescribed and over-the-counter / consumable.
During the development of such products and packaging, consideration needs to be given to many factors – not least optimal processing, engineered integrity, cost and usability.
However, a product can still fail prematurely, as the stresses of everyday use combine in unexpected ways to create new interactions.
Cambridge Medtech Solutions was asked to compare the robustness and usability of a range of competitive Lyophilised Products and their primary packaging, and to identify the failure modes.
We applied the Multiple Environment Over Stress Testing (MEOST) method, where the objective is to cause failures, by combining stresses beyond design limits.
In this way, an effective robustness assessment could be completed quickly using very small sample sizes, and any weak links in the design can be identified.
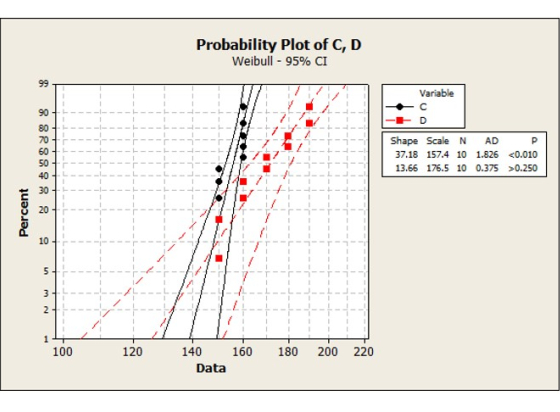
MEOST is primarily a qualitative test, but normalization of the over stress steps means that failure data can be plotted on a Weibull plot as %Stress*%Time.
This provided a semi-quantitative insight into how to maximise lifetime and reliability as part of life cycle management.
In the first round of testing, for some products the majority of failure modes were associated with the lyophilised product, whereas for other products the majority of failure modes were of the primary packaging. We then carried out a ‘mix and match’ round of testing to explore different combinations of lyophilised product and packaging.
Both rounds of testing were completed in less than one week. The study has provided valuable insights into the robustness and usability of these products.