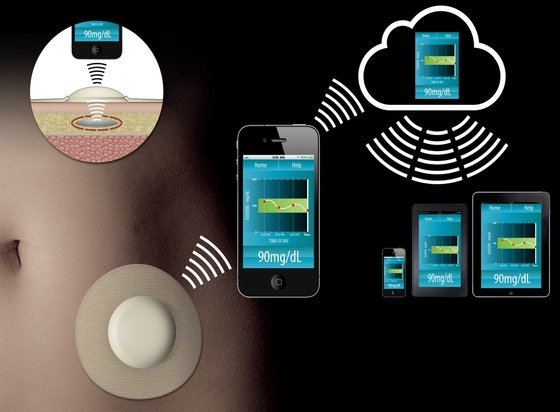
Continuous Glucose Monitor
Medical Device Development
Engineering, Industrial Design, Risk Management, Regulatory, Exploitation
The International Diabetes Federation (IDF) estimate that in 2011 approximately 366 million individuals worldwide have Type 1 or Type 2 diabetes mellitus, and that global prevalence will increase by 50% to 552 million individuals by 2030.
Diabetes accounted for at least $465 billion in global healthcare costs in 2011, or 11% of total healthcare costs in adults. This level of expenditure with the predicted increase in prevalence is unsustainable.
In the UK, NHS spending on diabetes was almost £14 billion in 2012, which was more than £2,500 per patient per year. This is £1.5 million per hour, or more than 10% of the NHS budget.
Jeffrey P. Koplan, a former Director of the US Centers for Disease Control and Prevention (CDC) said “Unless these dangerous trends are halted, the impact on our nation’s health and medical care costs will be overwhelming.”
There is no currently cure for diabetes, and diabetics have to manage their condition through insulin therapy, in association with self-monitoring of blood glucose (SMBG) – also known as finger stick blood testing.
Tight glycaemic control has been shown to reduce the likelihood of expensive, diabetes-related medical complications, such as kidney failure, cardiovascular disease, nerve damage, ulcers, stroke, blindness and amputation. However it is difficult for individuals to maintain this level of control, due to fear of hypoglycaemia, poor adherence to SMBG, and the episodic (non-continuous) nature of glucose level monitoring.
Real-time continuous glucose monitoring (CGM) provides a more complete picture of glucose levels over time, and in the context of daily activities. Importantly, it has been shown to be of clinical benefit to all insulin taking diabetics, and to reduce the likelihood of expensive complications.
However, despite these benefits, the current continuous glucose monitoring options available are not in widespread use, for reasons such as complexity, inappropriate expectations, discomfort, pain and risk of infection. Furthermore, the cost of the current CGM systems far out-weigh the likely cost savings, and so reimbursement occurs in only a small minority of cases.
Our client is developing a very low cost, non-invasive CGM system using an implant under the skin, which is regularly interrogated by a reader adhered to the skin, and the information will then be transmitted wirelessly to the user’s smartphone.
This CGM system is intended to deliver significant clinical benefits at a much lower cost, and be suitable for heathcare system reimbursement, thereby reducing overall health care system and social costs.
Scientific evidence to date shows that this implant technology indicates:
- Quick response time,
- Superior selectivity and sensitivity to glucose over lactate,
- Insensitivity to interferents,
- Meet the ISO 10993 and USP Class VI requirements for biocompatible materials,
- Will far exceed the ISO 15197:2013 requirements for accuracy.
Cambridge Medtech Solutions are responsible for all aspects of medical device engineering and design, risk management, and supporting the laboratory work, regulatory positioning and exploitation strategy.
This novel continuous glucose monitoring system is in pre-clinical development.
“We are very excited by this disruptive continuous glucose monitoring technology and the opportunity to deliver significant clinical benefits at a much lower cost. Cambridge Medtech Solutions have brought a wealth of medical device development experience to this programme, and we are delighted to be working with them.”