Organ Decellularisation and Cell Seeding
Pre-Clinical Research Bioreactor
Specification, Design, Build, Qualification
There is a world-wide shortage of donor organs for transplantation, and an emerging need to increase the patients’ quality and length of life and reduce healthcare costs.
One approach under investigation is to use decellularised organ scaffolds seeded with the patient’s own mesenchymal stem cells. This creates non-immunogenic, regenerative bespoke organ replacements.
Autologous cell seeding of the organ scaffold reduces the risk of organ rejection and removes the requirement for expensive immunosuppressant (anti-rejection) drugs that can have detrimental side effects on the patient and organ.
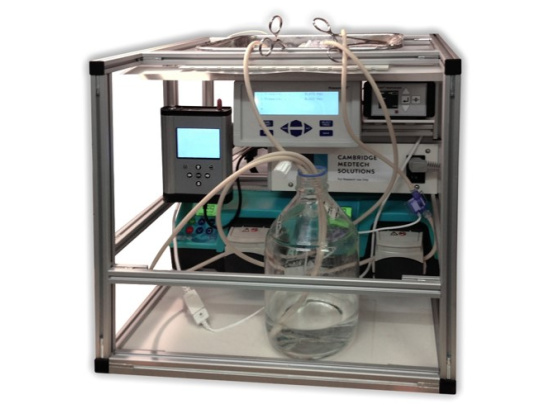
Based on initial studies, Cambridge Medtech Solutions were approached to design and build a pre-clinical research bioreactor for organ decellularisation and cell seeding.
This bioreactor enables the gentle removal of cells and cellular remnants from organ tissues. This leaves behind a purified biological scaffold that is cell compatible and retains the same characteristics as normal organ tissue.
The bioreactor can then be used to seed the acellular scaffold with the patients’ stem cells. Signals within the tissue trigger cell transformation which leads to recellullarisation.
To aid research, the bioreactor has the ability to operate multiple protocol variants, to operate over a temperature range of 5°C to 40°C, without any time limits.
The bioreactor is also capable of real-time and cumulative data monitoring of key physiological parameters during organ decellularisation and cell seeding.
“The expertise and prototype provided by Cambridge Medtech Solutions has allowed us to explore what platform technology would be needed to create bespoke tissue engineered seeded scaffolds.”
Head of R&D, Regenerative Medicine